Medical cannabis on the rise in Australia
As the medical cannabis industry continues to rapidly grow, compliance and security standards must evolve to ensure storage and distribution standards are met. In 2024, the industry was expected to be $1B, with scripts reportedly quadrupling since 2022. With such expedited growth occurring around medical cannabis, it is imperative the entire process from farming to manufacturing, through to distribution is safely secured to protect these schedule 8 drugs.
Protecting Schedule 8 Drugs
To protect medical cannabis you must first understand where the risk sits within the process of manufacturing and distribution, and what rules and regulations each step of the process must adhere to.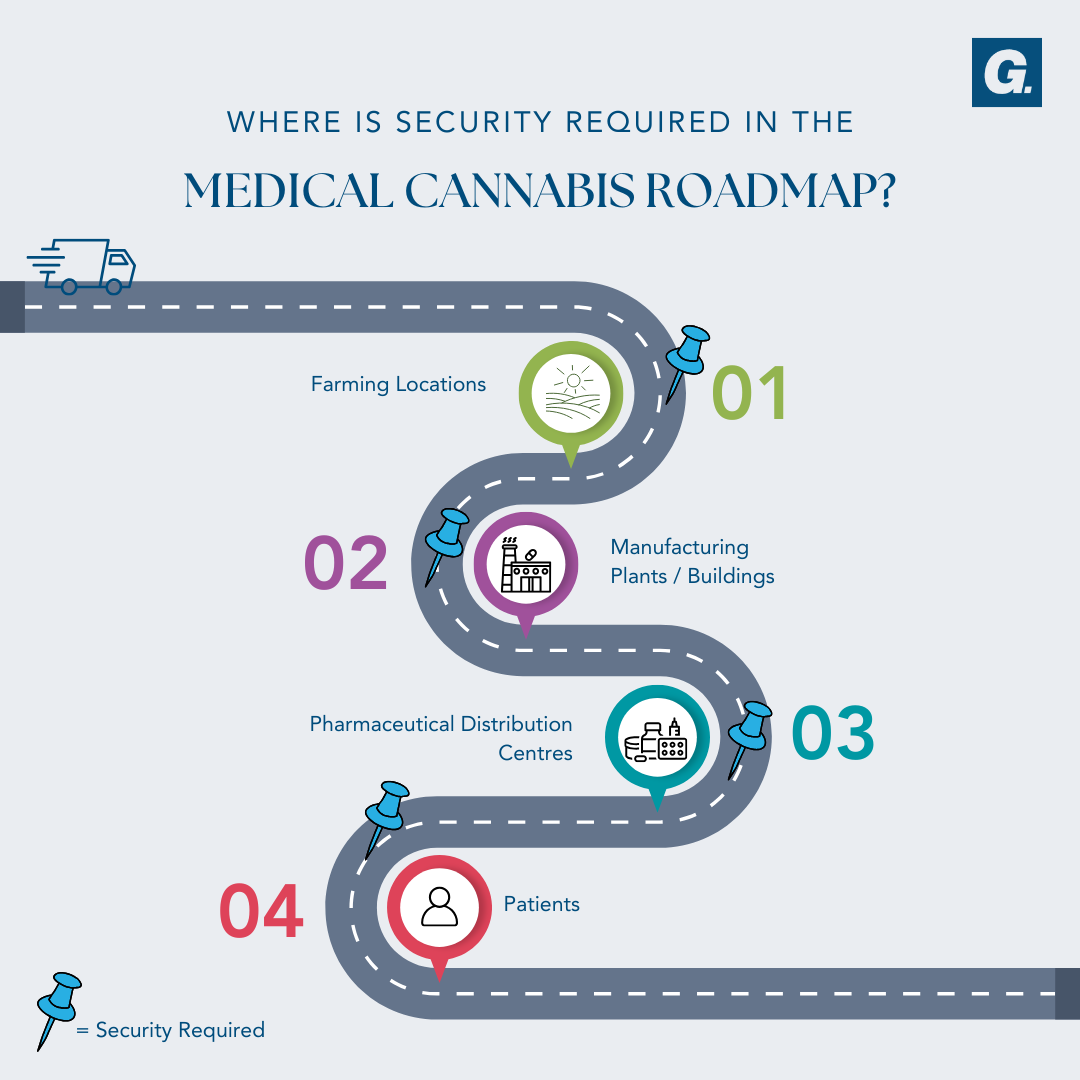
Throughout the manufacturing and distribution process, security is required at every checkpoint. While regular security measures such as CCTV cameras are a given at sites like these, other security touchpoints should be utilised to increase protection throughout.
Farming – These locations are extremely protected and on a need-to-know basis for security reasons. These locations should incorporate perimeter protection on the surrounds and building hardening / entrance control for any office locations.
Manufacturing Facility – These locations are usually discreet in nature to remain un-detected. Storage regulations are extremely important in these facilities, requiring vaults for the secure storage of goods. Other security measures that should be utilised in these locations are access controls, building hardening and safes.
Pharmaceutical Distribution Centre – Similarly to a manufacturing facility, distribution centres must store schedule 8 drugs accordingly. Using safes and vaults for drug storage, entrance control to manage access into secured areas and building hardening / hostile vehicle mitigation to protect against attacks is crucial in securing these facilities.
Transport Throughout / To Patient – The risk of goods in transit being attacked is thought to be higher than an attack on a facility due to accessibility. Due to medical cannabis being a schedule 8 drug and having a high illicit value, the risk surrounding transportation of goods increases. Safe and secure processes must be in place and to the standards of the ODC.
How Gunnebo Can Help
At Gunnebo, we have global experience in protecting high risk sites and assets, including pharmaceutical storage. Our product range can protect each phase of the manufacturing and distribution process, from the perimeter, down to the physical assets. We have listed the products which would be beneficial for these facilities to consider including in their security posture:
The Perimeter – Bollards, security barriers, security gates, and fencing.
The Building – Attack, blast or ballistic rated walling, windows and doors.
The Entrance – SpeedStiles, single person detection units, security booths, airlocks and turnstiles.
The Assets – Vaults, and document protection safes. * Gunnebo’s InstaVault meets all schedule 8 storage requirements.*
In Conclusion
Securing these facilities and Schedule 8 drugs isn’t just about compliance — it’s about safeguarding valuables and ensuring facilities are safe. Combining perimeter protection through to safe storage, facilities can mitigate risks and stay ahead of evolving threats. Prioritising security, medical cannabis providers can protect their staff, operations and patients who are reliant on these essential medical treatments.
For more information, contact us or connect with our Business Development Manager, Cristian Cerda, on LinkedIn.
